Abstract
Background: Therapy-related acute lymphoblastic leukemia (t-ALL) represents <7% of all ALL cases (Ganzel C, et al. 2015; 170:50-5). While treatment-related acute myeloid leukemia is more common and better described, our knowledge on t-ALL is limited. Furthermore, "secondary ALL" has been used as a broad term, combining patients with t-ALL and ALL patients with antecedent malignancy who did not receive chemotherapy or radiotherapy for the first malignancy (am-ALL). We investigated the clinical and molecular characteristics, as well as prognostic predictors of outcomes in t-ALL patients, while comparing t-ALL to am-ALL.
Methods: ALL patients treated at the Cleveland Clinic, University of Washington, University of Chicago, and Stanford University during the years 1997-2016 were screened for eligibility. Patients with a history of another malignancy prior to ALL diagnosis were included, and further categorized as t-ALL and am-ALL based on the administration of chemotherapy or radiotherapy for first malignancy. Patients with mixed lineage leukemia and CML in blast crisis were excluded. Complete response (CR) was defined as <5% blasts with an absolute neutrophil count (ANC) of >1000/uL and platelets >100,000/uL; CRi: same as CR without count recovery. Cytogenetic risk was ascribed by CALGB criteria. A subset of diagnostic samples were sequenced with libraries prepared according to Illumina's Nextera Rapid Capture Custom Enrichment Kit and subjected to massive parallel sequencing using the HiSeq 2000. Our panel consisted of 170 genes highly prevalent in hematologic cancers or associated with congenital blood disorders.
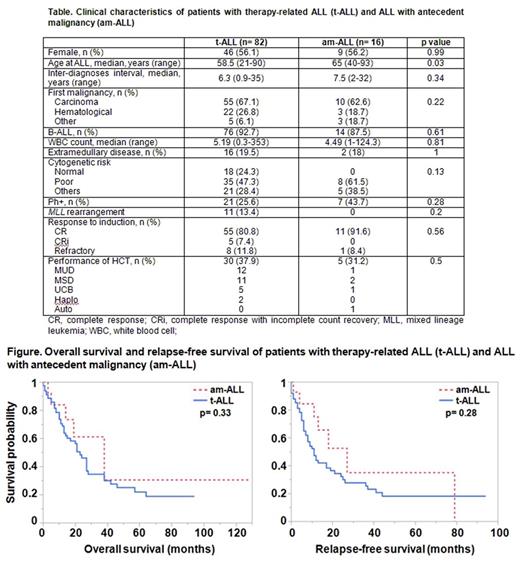
Results: A total of 82 patients with t-ALL, and 16 with am-ALL were identified. In the t-ALL group, 56.1% were female, 70.7% were Caucasian, 92.7% had B-ALL, and 47.3% had poor risk cytogenetics. The median age at ALL diagnosis was higher in the am-ALL group as compared to t-ALL (65 vs 58.5, respectively, p= 0.03), however the interval between the diagnosis of first malignancy and ALL was similar between the groups (7.5 vs 6.3 years, respectively, p= 0.34) (Table). In the t-ALL group, treatment for the first malignancy included alkylators in 64%, topoisomerase II inhibitors in 33%, and radiotherapy in 63%. The CR rate was significantly lower in patients with a history of radiotherapy (80% vs 100%, p= 0.003). Patients with t-ALL had a shorter median overall survival (OS) (23 vs 38 months, p= 0.33) and relapse-free survival (RFS) (11 vs 27 months, p=0.28) than patients with am-ALL, but the difference did not reach statistical significance (Figure). In univariable analysis, factors associated with worse outcomes in t-ALL patients included male sex (HR, 2.24; p= 0.009 for OS, and HR, 2.16; p=0.008 for RFS) and hematologic cancer history (HR, 2.14; p=0.03 for OS, and HR, 1.8; p= 0.06 for RFS), while Asian race (HR, 0.21; p= 0.04 for OS, and HR, 0.17; p= 0.01 for RFS) and performance of hematopoietic cell transplant (HCT) after ALL diagnosis (HR, 0.53; p= 0.04 for OS, and HR, 0.65; p= 0.15 for RFS) were associated with better survival. Other clinical factors analyzed, including age at ALL diagnosis, type of treatment for first malignancy, immunophenotype, and cytogenetics for ALL did not have a significant impact on survival. In multivariable analysis, longer interval between the first malignancy and ALL (HR, 0.93; p= 0.05 for OS, and HR, 0.94; p= 0.05 for RFS) and performance of HCT for ALL (HR, 0.44; p= 0.02 for OS, and HR, 0.61; p= 0.14 for RFS) were independent predictors of better outcomes in t-ALL. An expanded mutation panel was performed in 9 t-ALL and 3 am-ALL patients, for whom samples were available for sequencing. In the t-ALL group, all patients had at least one mutation: 3 patients had KDM6B , 2 patients had TP53 , and 2 patients had BRCA2 mutations. In am-ALL, 1 patient had JAK2 , and 1 patient had an NBN mutation.
Conclusion: Therapy-related ALL is a rare complication of treatment for previous malignancies. While there is no significant difference in clinical features of t-ALL vs am-ALL, our observation on genomic characteristics of a small cohort of patients suggest that they might be biologically different. Longer interval between two diagnoses and performance of HCT after ALL were associated with better outcomes. We believe that our results improve our knowledge on this rare entity, and should be further validated through larger prospective studies utilizing comprehensive lymphoid mutation panels.
Stock: Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Larson: Novartis: Consultancy, Research Funding; Amgen Inc.: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Astellas: Consultancy, Research Funding. Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees. Gerds: Incyte: Consultancy; CTI BioPharma: Consultancy. Maciejewski: Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Speaker Fees; Apellis Pharmaceuticals: Consultancy; Ra Pharma: Consultancy. Advani: Takeda/ Millenium: Research Funding; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.